MOFs Are Changing Medicine
In 2025, the Nobel Prize in Chemistry was awarded to Susumu Kitagawa, Richard Robson, and Omar M. Yaghi — for their pioneering work in the design and development of metal–organic frameworks. Their “molecular architectures contain rooms for chemistry,” as the Nobel Committee put it: metal ions serve as cornerstones, long organic linkers act as scaffolding, and together they assemble into crystalline networks filled with vast, tunable internal cavities.
This award is more than a symbolic crowning of decades of fundamental research — it also signals that MOFs are no longer esoteric curiosities, but a foundational materials platform whose breadth of applications extends from carbon capture to energy storage, and, importantly for us, to biomedical use.
For researchers working on bio-compatible drug delivery systems, this is a timely opportunity. The same properties that make metal-organic frameworks (MOFs) such appealing materials for gases or environmental chemistry – modularity, tunable porosity, and incredibly high internal surface area – also suggest new paradigms for how drugs can be formulated, delivered and controlled in the body.
In this blog post, we will look at how recent advances in recognition of MOFs for bio-applications fuel and invigorate the field of bio-MOFs in particular for drug delivery and the challenges that remain.
What Are Bio–MOFs — And Why They Are Special
In essence, MOFs are hybrid materials: the nodes are metal ions or clusters, the linkers are organic molecules, and they coordinate into a three-dimensional, periodic framework with internal pores. This is the classic topology that has now been proven by decades of research and recognized by the Nobel Prize.
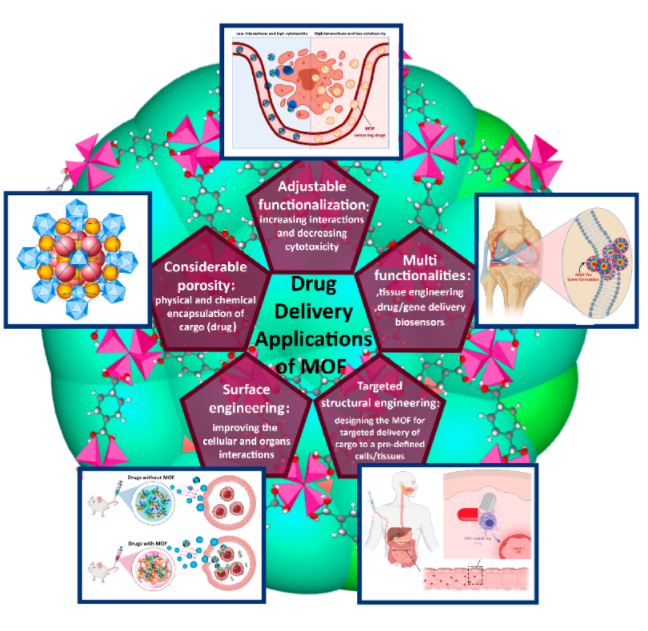
When these building blocks are chosen for biocompatibility — e.g., low-toxicity metal ions (Ca2+, Zn2+, Fe3+, K+, etc.) and biologically friendly linkers (amino acids, peptides, nucleobases, cyclodextrins, even drug molecules themselves) — the resulting structures are dubbed bio-MOFs. These frameworks combine the porosity and design-versatility of MOFs with the crucial requirement of biological safety.
Compared to “regular” MOFs (often designed for gas storage, catalysis, separation), bio-MOFs bring additional constraints — cytocompatibility, biodegradability or safe clearance, benign degradation byproducts — but also open up exciting possibilities: drug encapsulation; controlled release; targeted delivery; smart, stimuli-responsive behavior.
For instance:
- Amino acids or dipeptides can act as linkers, coordinating through their –COOH and –NH₂ groups, forming chelate rings with metal ions — achieving rigid or flexible frameworks with good biocompatibility.
- Nucleobases (e.g., adenine) bring multiple coordination sites and rigid scaffolds, offering a route to stable, biologically relevant frameworks.
- Cyclodextrins (natural cyclic oligosaccharides) have been used to build edible/ingestable MOFs (e.g., CD-MOF), which can load and release drugs — a direct bridge to oral or inhalable drug formulations.
Moreover — as you noted — it’s even possible to use certain active pharmaceutical ingredients (APIs) themselves (e.g., curcumin, non-steroidal anti-inflammatory drugs) as linkers, so the drug becomes part of the framework: a “drug-centered MOF.” This dual role (framework + cargo) is one of the most elegant and efficient drug-delivery paradigms.
Thus Bio-MOFs are not just carriers in the traditional sense — they are potentially integrated drug systems, where the chemistry of the carrier and the chemistry of the drug merge.
What the 2025 Nobel Prize Adds — New Energy, New Expectations
Why is the 2025 Nobel Prize relevant now? Here are a few reasons:
Legitimization of MOFs as a mainstream materials class. The Prize validates decades of MOF research and signals that MOFs are no longer fringe. For bio-MOFs, this may translate into more funding, more interest, more acceleration toward translational applications.
Focus on modular, tunable design. The award highlights the power of “reticular chemistry” — making frameworks by stitching molecular building blocks like Lego. This matches perfectly with the philosophy of designing bio-MOFs: choosing metal nodes and linkers with biological constraints but maximal design freedom.
Renewed optimism for scalable, real-world use. The original developers envisioned uses ranging from gas capture to catalysis. Now, with recognition and resources, engineers and biomedical scientists may push toward scale, reproducibility, safety — critical steps for eventual clinical translation of bio-MOFs.
In short: the Nobel is not the end of a story — it may well be the beginning of the next chapter, including biomedical translation.
Recent Trends & Opportunities: Bio–MOFs for Drug Delivery and Beyond
Bio-MOFs have already been explored in drug delivery — but the field appears to be accelerating. Below are some of the most promising trends and opportunities.
- Bone Tissue Engineering & Bone Regeneration
MOFs are emerging as novel platforms for bone repair and regeneration. Due to their high surface area, tunable ion release (e.g., Ca2+, Zn2+, Mg2+), and porous structure, MOFs can mimic aspects of bone mineral matrix, provide scaffolding for cell growth, and deliver therapeutic ions or drugs to support osteogenesis — while possibly incorporating antibacterial functionality to prevent infection.
- Drug Encapsulation — Oral, Pulmonary, Even Inhalable Therapies
Bio-MOFs offer flexible platforms for encapsulating small molecules, poorly soluble drugs, or drugs that require protection/stabilization. As previously demonstrated (e.g., CD-MOF systems), MOFs can enhance solubility and stability, potentially improving bioavailability.
Especially exciting is the prospect of inhalable MOF-based dry powders for pulmonary delivery. Low-density, porous MOF particles can achieve aerodynamic diameters suitable for lung deposition — opening routes for local lung therapy, or even systemic absorption via pulmonary delivery.
- Controlled, Stimuli–Responsive and Targeted Release
Because of their modular chemistry, bio-MOFs can be engineered to respond to external stimuli — pH, redox state, enzymes, even magnetic field or light (with functionalization) — allowing for controlled or “smart” release. For instance, frameworks stable at physiological pH but degrade or open pores under acidic tumor microenvironments, or in response to intracellular triggers.
Combining this with surface modifications (e.g., PEGylation, targeting ligands) creates a powerful toolbox for targeted, controlled, and efficient drug delivery.
- Multi–Functional “Theranostic” Platforms
By embedding imaging agents (fluorophores, MRI contrast metals), therapeutic APIs, and targeting moieties into a single bio-MOF, one can envision “theranostic” platforms — systems that diagnose and treat, or that allow tracking of drug delivery, release kinetics, biodistribution, and clearance.
Given the modularity demonstrated by MOF pioneers, building such complex, multifunctional Bio-MOFs is well within reach.
Challenges — Why Bio–MOFs Are Still Mostly in the Lab
Despite all the enthusiasm, bio-MOFs for drug delivery remain largely at the research stage. Several key challenges must still be addressed before clinical translation.
- Biocompatibility and Safety: It is essential to thoroughly evaluate acute and long-term toxicity, immunogenicity, biodegradation, and fate of both metal ions and linkers. Without robust in vivodata, regulatory approval remains distant.
- Pharmacokinetics (ADME): Absorption, distribution, metabolism, and excretion (ADME) of MOF materials need comprehensive study. For many bio-MOFs, data on biodistribution, clearance routes, and possible accumulation or long-term retention is lacking.
- Control of Drug Loading & Release: While molecular flexibility is a strength, it also introduces variability. The strength of host–guest interactions (electrostatic, hydrogen bonding, π–π, etc.), pore collapse, framework degradation — all influence loading efficiency and release profile. Achieving reproducible, predictable behavior is nontrivial.
- Scalable, Reproducible Synthesis: Lab-scale syntheses (hydrothermal, solvothermal, mechanochemical, etc.) may not translate readily to GMP-compliant production. Purity, batch-to-batch reproducibility, residual solvents or unreacted linkers/metal ions — all present practical challenges for pharmaceutical use.
- Regulatory and Quality Control: As a novel excipient / carrier class, bio-MOFs would require new guidelines: for manufacturing, quality control, impurity profiling, stability, storage, sterility, etc. Regulatory agencies may not yet have a clear pathway for MOF-based drug products.
In short — the gap between “promising nanomedicine research” and “approved clinical drug product” remains significant.
What the Post–Nobel Future Could Look Like
With the 2025 Nobel Prize shining a spotlight on MOFs, I expect several developments that could accelerate bio-MOF research — and perhaps push it toward real-world applications.
- Increased Funding and Cross–Disciplinary Collaboration
Materials scientists, chemists, biomedical engineers, and pharmaceutical scientists will likely be drawn together. Funding agencies may prioritize MOF-based translational research (drug delivery, tissue engineering, water harvesting for global health, etc.).
- Commercial Translation and Pilot Platforms
Just as companies are now scaling MOF-based water harvesters or gas-capture modules, biotech firms (or academic-industrial partnerships) could begin pilot production of bio-MOF-based drug carriers — especially for niche applications (e.g., inhalable dry powders, bone scaffolds, localized cancer therapy).
- Standardization and Regulatory Framework Building
To move beyond academic studies, communities will need to develop standard protocols: purity criteria, toxicity evaluation guidelines, stability testing, and manufacturing standards — paving a regulatory path.
- Rational Design of “Next–Generation” Bio–MOFs
Leveraging the “reticular chemistry” principle, researchers may design highly sophisticated bio-MOFs: multi-functional, stimuli-responsive, biodegradable, even programmable. The modularity allows for combinatorial exploration of metal–linker combinations, pore sizes, surface chemistries, and functional groups — akin to synthetic biology but in the materials realm.
Why Bio–MOFs Deserve Attention in Drug Delivery
Bio-metal-organic frameworks may appear to overlap with liposomes, polymer nanoparticles, and gene delivery systems—but they are more of a compelling complement. Here’s why:
- They offer a third paradigm beyond liposomes and polymeric nanoparticles: a rigid (or semi-rigid) inorganic–organic “solid scaffold” with high porosity and design flexibility. This could overcome some limitations of soft carriers (e.g., leakage, stability) while retaining tunability.
- Bio-MOFs may enable co-delivery or multi-drug formulations in a single carrier, especially using drugs themselves as linkers — an efficient and elegant design.
- For hard-to-deliver drugs (poorly soluble, unstable, volatile), the confinement inside MOF pores may provide stabilization, protection and controlled, slow release — improving pharmacokinetics and bioavailability.
- For specialized delivery routes (pulmonary, bone implantation, local therapy), the mechanical and structural flexibility of MOF-based carriers may provide advantages over conventional carriers.
In short — bio-MOFs could enrich the “drug delivery toolbox,” offering a fundamentally different platform that may fill niches where liposomes or polymeric NPs are less optimal.
Conclusion
The Nobel Prize for MOF pioneers is a clear indication that the science community – and the world at large – believes in the power of MOFs to change the world. For drug delivery researchers, it should be seen as an inspiration – and a challenge, rather than just a homage. Bio-MOFs are at the confluence of material sciences, coordination chemistry, pharmacology and biomedical engineering. The necessary components are all present: modular design, biocompatible building blocks, tunable porosity, diverse synthetic strategies. What remains to be done is judicious development: careful biological evaluation, scalable manufacturing, regulatory pathway development, and, most importantly, proof of compelling therapeutic advantages.
If those steps are taken, bio-MOFs could become a new generation of drug carriers — perhaps even paradigm-shifting ones. And now, with MOFs in the spotlight more than ever, the momentum might just be building for that leap.
Reference
1. Mohammad Reza Saeb, et al.; Metal-Organic Frameworks (MOFs)-Based Nanomaterials for Drug Delivery. Materials. 2021, 14(13), 3652
| Cat. No. | Product Name | MW | |
| CDM-CH032 | PCN-333 (Al) | 1026.72 | INQUIRY |
| CDM-CH1572 | UIO-66 (Ce) | 1953.38 | INQUIRY |
| CDM-CH230 | MIL-101 (Cr) | 719.36 | INQUIRY |
| CDM-CH251 | ZIF-67 | 223.14 | INQUIRY |
| CDM-CH231 | (CuI)4 (DABCO)2 | 986.15 | INQUIRY |
| CDM-CH008 | MIL-124 (In) | 505.79 | INQUIRY |
| CDM-CH265 | PCN-250 (Fe) | 1535.83 | INQUIRY |
| CDM-CH249 | KAUST-7 | 458.80 | INQUIRY |
| CDM-CH281 | NH2-MIL-125 (Ti) | 1653.74 | INQUIRY |
| CDM-CH1571 | MIL-47 (V) | 231.06 | INQUIRY |
| CDM-CH272 | IRMOF-3 | 814.95 | INQUIRY |
