Cracking the Parkinson’s Puzzle with Animal Models
Parkinson’s disease (PD) is a complex neurological disorder which scientists have been trying to understand and treat for many years. The disease causes patients to experience tremors and rigidity and slow movement while also producing various non-motor symptoms that include sleep problems and constipation. The disease develops slowly through time while it steals away the body’s ability to function normally. Scientists need to solve biological mysteries which occur during the slow process of development. One of their most powerful tools? Animal models.
Over the past decade, remarkable progress has been made in developing experimental models that mimic PD in animals. From toxin-induced rodent models to gene-edited monkeys and even brain organoids grown from human stem cells, these models help researchers understand what really happens inside the Parkinsonian brain — and how we might one day stop it.
The Dopamine Connection and the Search for a Perfect Model
The hallmark of Parkinson’s disease is the loss of dopamine-producing neurons in a brain region called the substantia nigra. Without dopamine, the brain’s communication with muscles falters, leading to the characteristic motor impairments. Yet, PD isn’t just a “dopamine problem.” Inflammation, mitochondrial damage, and abnormal protein buildup — especially of alpha-synuclein — all contribute to the disease’s progression.
That complexity makes it nearly impossible for one animal model to capture everything about PD. Instead, scientists rely on different types of models, each designed to highlight a specific aspect of the disease.
- Neurotoxin-Induced Models: The Classic Workhorses
The earliest PD models were based on neurotoxins — chemicals that specifically damage dopamine neurons. MPTP and 6-hydroxydopamine (6-OHDA) serve as fundamental laboratory tools for neuroscience researchers to conduct their studies. The compound MPTP which caused Parkinson-like symptoms in drug users during the 1980s enters the brain through the blood-brain barrier to destroy dopaminergic neurons in mouse and primate models. Scientists use 6-OHDA to create brain conditions that resemble oxidative stress by causing dopamine neuron death in the brain.
Other environmental toxins such as rotenone (an insecticide), paraquat, and maneb (both herbicides) have also been used to create PD-like conditions. The models show potential connections between human Parkinson’s disease development and environmental pesticide and heavy metal exposure.
The advantage? They are reproducible and simple to create. The downside? They don’t form Lewy bodies — the protein aggregates made of alpha-synuclein that are the pathological signature of PD. So while these models simulate the symptoms, they miss a key piece of the puzzle.
- Genetic Models: Engineering Parkinson’s in the DNA
The field of genetics led scientists to identify SNCA and LRRK2 and PINK1 and Parkin and GBA as genes which contribute to Parkinson’s disease. The three genes function in different cellular pathways which include mitochondrial operation and protein elimination and their mutations lead to premature or inherited Parkinson’s disease.
Scientists study brain function changes through animal gene modifications that introduce particular mutations. The SNCA A53T mutation in mice leads to excessive alpha-synuclein production which results in Lewy body-like protein clumps that match human disease symptoms. The LRRK2 gene mutations result in lysosomal breakdown and disrupted neuronal communication but GBA mutations lead to lipid processing defects which increase the risk of alpha-synuclein accumulation.
These genetic models are invaluable for testing gene therapies and exploring molecular mechanisms. However, they often lack one frustrating feature — significant dopamine neuron loss. They show the “cause” of PD, but not always the full-blown “disease.”
- Alpha-Synuclein Models: Recreating PD’s Toxic Signature
Since alpha-synuclein aggregation is at the heart of Parkinson’s pathology, newer models directly target this protein. Scientists use preformed fibrils (PFFs) which contain misfolded alpha-synuclein seeds to inject into animal brains which then causes a chain reaction of protein misfolding that moves through the nervous system in a manner similar to an infection.
Scientists use recombinant adeno-associated viruses (rAAV) as viral vectors to transport mutant alpha-synuclein genes into particular brain regions. The models successfully duplicate Lewy body development and disease progression pattern which provides scientists with an accurate understanding of Parkinson’s disease advancement.
These “synucleinopathy” models are also crucial for testing drugs that block protein aggregation or enhance its clearance through cellular cleanup systems like autophagy.
- Combination Models: Capturing the Full Picture
Because Parkinson’s disease involves both genetic and environmental factors, scientists are now blending the two. For instance, exposing DJ-1 knockout mice to MPTP toxin causes more severe neuron loss than either approach alone. Combining alpha-synuclein fibril injections with low-dose MPTP enhances protein propagation and mimics both the genetic and environmental contributions to PD.
These hybrid models better represent the multifactorial nature of the disease and provide a versatile platform for testing neuroprotective and disease-modifying therapies.
- Non-Motor Models: Beyond Tremors and Rigidity
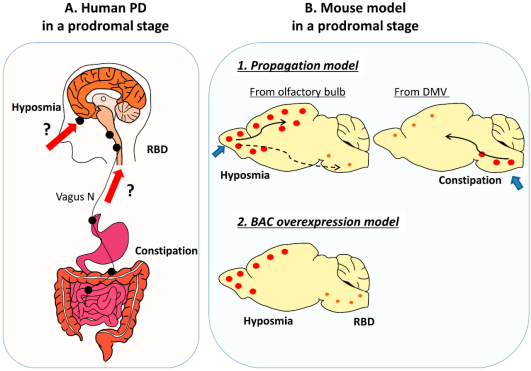
Interestingly, non-motor symptoms such as constipation, loss of smell, and REM sleep behavior disorder often appear years before the first tremor. To study these, researchers developed models that trace the disease’s path from the gut to the brain — a concept known as the “gut-first hypothesis.”
By injecting alpha-synuclein fibrils into the intestines of rodents, scientists have shown that pathology can travel along the vagus nerve to the brainstem, mirroring early-stage human PD. Such models may help identify early biomarkers, opening doors for pre-symptomatic diagnosis and intervention — something medicine desperately needs.
- Non-Human Primates: The Gold Standard
Rodent models are convenient, but their brains differ from ours. The human body shares multiple similarities with non-human primates including macaques and marmosets because they possess similar anatomical structures and neurochemical makeup and sleep patterns. MPTP-treated monkeys develop Parkinson-like tremors and rigidity which matches the human Parkinson symptoms.
The use of primate models as research tools provides the most direct path from laboratory studies to human clinical testing although they come with high costs and raise significant ethical concerns. They help scientists fine-tune new treatments — from stem cell transplants to gene therapy — before testing them in humans.
- Emerging Models: From Stem Cells to Mini-Brains
In the era of precision medicine, animal models are now being complemented — and sometimes replaced — by human-based systems. Using induced pluripotent stem cells (hiPSCs), scientists can reprogram a PD patient’s skin cells into dopamine neurons that carry their unique genetic mutations. These lab-grown neurons display the same pathological features seen in patient brains, making them ideal for personalized drug screening.
Even more exciting are midbrain organoids — miniature, 3D brain-like structures that produce dopamine and mimic human brain development. Researchers can introduce PD-related mutations like PINK1 or GBA into these organoids to observe how pathology unfolds in real time. Such models are bringing us closer than ever to “disease-in-a-dish” platforms for testing novel treatments.
Looking Ahead: Toward Personalized Parkinson’s Medicine
Despite tremendous progress, no model perfectly replicates human PD. Each captures a piece of the puzzle — whether it’s neuroinflammation, protein aggregation, or genetic predisposition. The future likely lies in combining approaches: merging gene-editing technologies like CRISPR with hiPSC-derived neurons, organoids, and even optogenetic tools that can control neural activity with light.
Together, these cutting-edge systems are transforming how we study, diagnose, and treat Parkinson’s disease — not as a single disorder, but as a complex syndrome with many paths and possibilities. The ultimate goal? To turn understanding into healing and, one day, stop the silent march of Parkinson’s altogether.
Related Services
