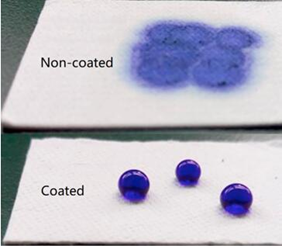
What are Functional Biomedical Coatings?
Functional biomedical coatings are specially engineered surfaces on biomedical materials that enhance biocompatibility and other functional properties through advanced surface modification technology. These coatings find broad application within medical settings including uses for implants as well as in vitro auxiliary devices and diagnostic equipment.
The main functions include:
Antibacterial function: Medical professionals can prevent bacterial adhesion and reproduction using contact antibacterial coatings, anti-adhesion antibacterial coatings or intelligent antibacterial coatings to reduce medical-induced infection risks. Tetra ammonium salts together with antimicrobial peptides and nanometal materials give antibacterial coatings their ability to efficiently damage bacterial cell membranes.
Anti-adhesion function: Hydrophilic polymer coatings as surface modification techniques block non-specific biological molecule adherence to surfaces and minimize biofilm development.
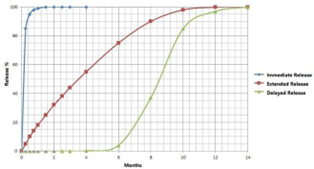
Drug controlled release function: The coating provides controlled drug release which enhances therapeutic outcomes and minimizes side effects. The coating embeds drug molecules using layer-by-layer self-assembly technology to create a local and sustained drug release mechanism.
Biosensing function: Sensor coatings can improve detection of biomarkers and body environment monitoring.
Promoting cell behavior: Functional coatings allow tissue engineering to benefit from controlled cell behaviors including adhesion along with migration and proliferation. Materials including polydimethylsiloxane (PDMS) and hyaluronic acid (HA) serve to enhance cell adhesion and proliferation capabilities.
What are the Benefits of Applying Functional Coatings to Medical Device Surfaces?
Despite rapid advancements in medical technology modern medical devices encounter numerous severe challenges which functional coating technology breakthrough application helps to solve. Medical devices that do not possess functional coatings can encounter multiple risks and defects:
Immune response and tissue rejection: Medical devices lacking functional coatings behave as foreign objects to patients which activates their immune systems and results in inflammation and infection that can lead to device failure. The absence of a protective barrier can result in scar tissue formation which interferes with the proper operation of the device.
Functional failure: The absence of protective coatings can lead to functional failure through material aging and bacterial contamination resulting from broken oxygen tubes or loose connections.
Risk of infection: Medical devices that lack coatings fail to prevent bacterial contamination resulting in higher infection risks. Bacterial infections can originate from medical nebulizers which experience improper packaging and material aging.
Mechanical performance issues: Medical devices without coatings experience mechanical property deterioration such as reduced strength and increased surface roughness which leads to decreased service life and safety performance.
Biocompatibility issues: Without protective coatings medical devices can cause adverse or allergic reactions in patients.
Risk of improper operation: Medical devices without protective coatings become difficult to use as they miss essential lubrication and protective layers which increase patient risks of discomfort and injury.
Long-term stability issues: Medical devices without coatings may experience performance degradation during long-term use, such as coating shedding, particle release, etc., which may lead to serious adverse events such as thrombosis and tissue necrosis.
Functional coating technology is systematically breaking through these bottlenecks through precise design at the molecular level. To address the infection problem, the nanosilver/zinc oxide composite coating can reduce the bacterial colonization rate on the catheter surface by 99.6%, and its sustained release mechanism can last for more than 28 days.
To solve the problem of thrombosis, the bionic heparin-albumin bilayer coating prolongs the thrombosis time of blood-contacting devices by 3 times, while avoiding the risk of bleeding from systemic anticoagulation.
In terms of promoting tissue regeneration, the micro-nanostructured hydroxyapatite coating can increase the adhesion density of bone cells by 5 times, and combined with the gradient release of BMP-2 growth factor, the bone integration cycle can be shortened to 6-8 weeks.
For the surface modification of precision instruments, the super-lubricating polydopamine coating reduces the friction coefficient of the nerve catheter to 0.02, significantly reducing the risk of tissue damage. More noteworthy is the breakthrough of intelligent response coatings, such as pH-sensitive antibacterial coatings that can trigger burst release of drugs in the acidic environment of the infection site, achieving precise strikes on the infected area.

These technological innovations not only extend the service life of medical devices by an average of 2-3 times, but also promote the development of personalized medicine - the multifunctional gradient coating constructed by 3D printing technology has achieved the spatiotemporal synergy of antibacterial, pro-angiogenic and anti-calcification functions.
With the advancement of molecular self-assembly and atomic layer deposition technology, coatings will continue to evolve in the direction of thinner (submicron level), smarter (biosignal response), and more environmentally friendly (water-based preparation), opening up a new dimension for improving the performance of medical devices.
What are the Common Material Compositions of Functional Biomedical Coatings?
| Category | Material Composition | Key Parameters | Function | Applications | Additional Parameters |
|---|---|---|---|---|---|
| Polymer Materials | Polyethylene Glycol (PEG), Polydopamine, PLGA | Hydrophilicity, biodegradability (e.g., PLGA degrades in 1–6 months) | Anti-adhesion, drug delivery | Catheters, surgical tools | Thickness: 10–500 nm; Biocompatibility: ISO 10993 compliant |
| Nanomaterials | TiO₂, Silver Nanoparticles (Ag NPs) | Particle size (10–100 nm), antimicrobial efficiency (>99% for Ag NPs) | Antimicrobial, antiviral | Implant surfaces, wound dressings | Toxicity threshold:<50 ppm Ag; UV activation required for TiO₂ |
| Bioceramics | Hydroxyapatite (HAp), Calcium Silicates | Porosity (30–70%), compressive strength (50–150 MPa) | Osteoconduction, biointegration | Orthopedic/dental implants | Degradation rate: 0.1–1 μm/year (HAp); pH sensitivity: stable in physiological conditions |
| Metallic Materials | Ti Alloys, Co-Cr Alloys, Mg Alloys | Corrosion resistance (e.g., Mg alloys degrade in 6–12 months in vivo) | Load-bearing, biocompatibility enhancement | Bone screws, stents | Surface roughness: Ra < 1 μm; Adhesion strength: >20 MPa |
| Biomolecules | Antimicrobial Peptides, Heparin, Hyaluronic Acid | Molecular weight (e.g., Heparin: 3–30 kDa), binding affinity | Anticoagulation, anti-inflammatory | Blood-contacting devices | Stability: 6–12 months shelf life; Temperature sensitivity: store at 2–8°C |
| Functional Polymers | Zwitterionic Polymers, PVDF, PMMA | Charge density (e.g., zwitterionic: ±1.2 C/m²), thermal stability (>150°C for PVDF) | Anti-fouling, biosensing | Diagnostic devices, sensors | Dielectric constant: PVDF ≈ 8–13; Optical transparency: PMMA > 90% |
| Composites | HAp-PLA, PLGA-Silver NP Blends | Hybrid ratio (e.g., 60% HAp + 40% PLA), synergistic antimicrobial/mechanical effects | Multifunctional performance enhancement | Bone grafts, antimicrobial coatings | Interfacial bonding strength: 15–30 MPa; Thermal expansion: 5–10 ppm/°C |
| Special Coating Tech | Laser-Clad Carbides (e.g., SiC), Oxide Coatings (Al₂O₃) | Hardness (e.g., SiC: 2500–3000 HV), wear resistance (10⁻6mm3/N·m) | Extreme durability, corrosion resistance | Joint replacements, industrial implants | Processing temperature: 1500–2000°C; Coating-substrate mismatch:<5% strain |
Why Choose Our Functional Biomedical Coating Services?
Excellent Biocompatibility, Reducing the Risk of Adverse Reactions
Carefully selected bio-inert materials
Advanced surface modification technology
Powerful Antibacterial Performance, Protecting Patient Safety
Innovative antibacterial coatings (such as, sustained-release antibacterial drugs and contact sterilization materials)
Long-lasting and efficient antibacterial effect
Customizable Functional Design to Meet Diverse Needs
Customized according to your specific application

Anti-calcification coatings of implants.
Drug eluting coatings
Flexible process selection (Such as, plasma spraying, sol-gel method, and electrochemical deposition)
Strict Quality Control System Maintains High Product Standards
Full-process quality monitoring
Industry-compliant certification
Professional Technical Team and Comprehensive Technical Support
Experienced R&D team
All-round technical support
Instrument Platform
In order to provide high-quality medical device coating solutions, we have a comprehensive and advanced instrument platform to support our coating services. This includes special instruments and equipment corresponding to PVD, CVD, electroplating and spraying technologies involved in customized coatings, such as vacuum coating equipment, multi-layer deposition equipment, chemical vapor deposition furnace, PECVD equipment, LPCVD equipment, and tubular CVD equipment, etc.; in addition, we also have an instrument platform for medical device coating quality testing, including: coating thickness measuring instrument, bonding strength tester, and surface roughness tester, etc.
Service Process
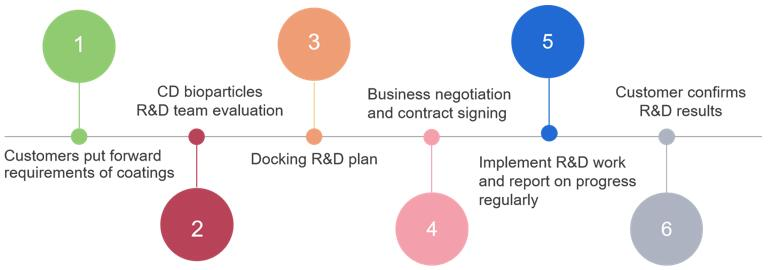
What are functional biomedical coatings, and what are their core functions?
Functional biomedical coatings are specialized surface layers applied to medical devices via surface modification technologies. They enhance biocompatibility, antimicrobial activity, anti-adhesion, drug-controlled release, and other critical properties. Key functions include:
Antimicrobial action (e.g., nanosilver coatings eliminate 99% of pathogens); Antithrombotic properties (e.g., heparin-based coatings triple thrombosis resistance time);
Tissue regeneration promotion (e.g., hydroxyapatite coatings shorten bone integration to 6–8 weeks);
Smart responsiveness (e.g., pH-sensitive coatings trigger drug release in infected areas);
Mechanical protection (e.g., silicon carbide coatings with hardness of 2,500–3,000 HV improve wear resistance).
Why are functional coatings essential for medical devices? What risks arise without them?
Functional coatings address critical risks in uncoated devices:
Infection: Catheters without antimicrobial coatings show 10× higher bacterial colonization rates.
Immune rejection: Untreated implants may trigger inflammation or fibrosis (e.g., titanium alloy implants).
Mechanical failure: Uncoated joint replacements with high friction coefficients (>0.5) wear out prematurely.
Drug inefficiency: Systemic drug delivery achieves 10–20% efficacy vs. 80%+ with localized controlled-release coatings.
Degradation: Uncoated magnesium alloy implants degrade fully within 6–12 months, risking structural collapse.
How to select coating materials based on application scenarios?
Prioritize materials aligned with device type, function, and biocompatibility:
Orthopedic implants: Hydroxyapatite (HAp) composites (5× higher osteoblast adhesion) or PLGA-silver nanoparticle blends (dual antimicrobial/mechanical support).
Cardiovascular stents: Heparin-albumin bilayers (antithrombotic) or sirolimus-eluting coatings (prevent restenosis).
Diagnostic tools: Zwitterionic polymers (anti-fouling) or PMMA (optical transparency >90%).
Catheters: Lubricious polydopamine coatings (friction coefficient<0.02) or pH-responsive antimicrobial layers.
How to validate coating biocompatibility and durability?
Rigorous testing ensures compliance and performance:
Biocompatibility: ISO 10993 tests for cytotoxicity (e.g., >80% L929 cell viability in extract solutions).
Mechanical stability: Adhesion strength (>20 MPa), wear resistance (wear rate<10-6 mm3/N·m), and friction coefficient (<0.1).
Antimicrobial efficacy: >99% inhibition of Staphylococcus aureus per ASTM E2180.
Long-term stability: Accelerated aging tests (e.g., 1,000-hour humidity cycles with no delamination).
Drug release profiling: HPLC validation of zero/first-order kinetic models.